We can define diffusion as the movement of atoms. In another words, matter is transported inside matter.
But diffusion has rules.
Atoms will only move from a region of higher concentration to a region with lower concentration.
It is also important to define what diffusion is not:
Diffusion is not the general flow of matter (like in a river). It is a gradual (dispersion) movement of individual atoms.
Also do not confuse diffusion with osmosis.
Both process want to equalize the concentration. They are also passive and will occur naturally.
However...
Osmosis is a one way, solvent only transfer of matter. Diffusion is a transfer it all mechanism, not just limited to liquid phase.
So you may consider osmosis as a special case of diffusion.
Why is diffusion important?
Diffusion is key to understand a material's micro structure. Nucleation and grain growth occurs during solid state diffusion.
Recrystallization is another example. Deformed grains are replaced by new defect-free grains that nucleate and grow until the original grains are gone.
Why diffusion occurs?
In short, diffusion occurs because matter is not evenly distributed. But let's understand it from an atom's perspective.
Atoms are in constant movement. They only stop at the absolute zero.
While they are vibrating they may occasionally find a place with no atoms. They may also find empty spaces that are big enough to accommodate them.
So in time they will fill the voids more evenly.
What affects the rate of diffusion?
High concentration means higher rate of diffusion.
So naturally, diffusion continues until there is no more difference in concentration.
But it is not just the concentration gradient that affects diffusion speed.
Temperature
Temperature is also an important factor.
Temperature is a measurement of an atom's vibration. The more an atom vibrates the more chance he has to randomly find a new empty spot to be.
Pressure
May be caused by internal or external stresses (forces).
Recrystallization is an example of diffusion caused by internal stresses.
Creep is an example of diffusion caused by external stresses.
Atom size
Big atoms will have a hard time finding room among smaller atoms. The opposite is not true.
Small atoms will find its way in to another material and get trapped in it.
An example is carbon in iron. It gets trapped in between iron atoms. We call it steel.
Small atoms like hydrogen (H0) can get caught during welding. During cooling they may combine to hydrogen gas (H2) and crack the weld.
Atomic bonds
An atom will need to break its current atomic bond first, before being able to wander around.
Therefore strong atomic bonds will require much more energy (or temperature) than weaker atomic bonds.
Example: An ionic bond will require much more energy than a metallic bond.
How to calculate diffusion rate
According to Fick's law, the diffusion rate is:
- J is the quantity and direction of transfer.
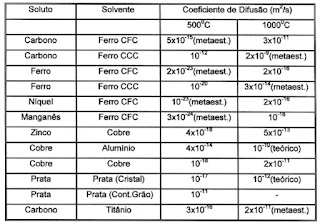
- D is the atomic diffusion coefficient. Check the table for a few examples.
- The ratio represents the concentration difference.
Note 1: The coefficient "D" is dependent on the atoms (Like carbon and Iron) but also on the temperature.
Note 2: Fick's second law take into account that concentration changes all the time.
How to speed up diffusion
Diffusion is time dependent but it can be optimized.
You can change the alloy in the first place and choose one with higher diffusion rate. That's what you can do if you are in the project phase.
Also thin materials will diffuse faster than thick ones. So if your base material has a lot of thickness it will take more time to heat treat it.
If you are in the production stage you can increase the temperature when possible.
Citation
When you need to include a fact or piece of information in an assignment or essay you should also include where and how you found that piece of information (Diffusion).
That gives credibility to your paper and it is sometimes required in higher education.
To make your life (and citation) easier just copy and paste the information below into your assignment or essay:
Luz, Gelson. Diffusion. Materials Blog. Gelsonluz.com. dd mmmm. yyyy. URL.
Now replace dd, mmmm and yyyy with the day, month, and year you browsed this page. Also replace URL for the actual url of this page. This citation format is based on MLA.




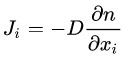





COMMENTS