The properties of any material depends on how its atoms are arranged.
Crystal structure
Most materials that we use have a repetitive pattern that we call crystal structure. So a crystalline material will have its atoms organized and repeated in all three dimensions.
Liquids and gases are not crystals. They don't have a repeating pattern of atoms.
Examples of crystals include diamonds, but also sugar and salt.
In total there are seven crystal systems:
- Cubic
- Tetragonal
- Orthorhombic
- Rhombohedral
- Hexagonal
- Monoclinic
- Triclinic
Note: The rhombohedral and hexagonal are both hexagonal but are two different crystal systems. That's why some say we have 6 crystal families.
Unit cell
The unit cell is the minimum structure that still maintains the material's properties. It's the building block that makes the material.
Bravais has described where each atom goes in the structure.
Any crystalline system can be described using one of the 14 bravais structures.
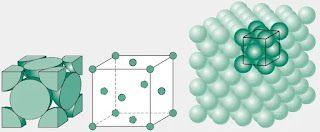
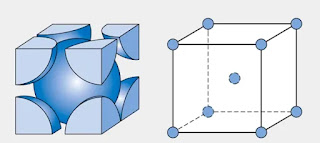
Most metals exist in nature as:
- Face centered cubic (FCC)
- Body centered cubic (BCC)
- Hexagonal close packed (HCP)
Face centered cubic
Body centered cubic
Hexagonal close packed (HCP)
Periodic table of unit cells
The highlighted in green are the HCP elements.
Tip: Click it to enlarge.
Atomic Packing Factor
Atomic packing factor is the ratio of atomic volume in a unit cell volume.
It's a way of saying how many atoms are actually in a unit cell.
The atomic packing of the most common unit cells are:
- FCC = HCP = 74% (26% void space in unit cell)
- BCC = 68%
Polymorphism and alotropy
Atoms have alotropic forms and so does unit cells.
And that makes alotropy very important to any engineer.
As some materials changes its forms we can use it to our advantage.
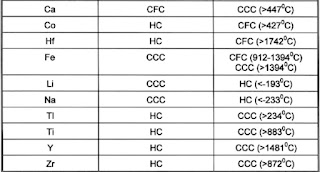
The classical example is steel where it changes from BCC to FCC and back to BCC. All depending on the temperature and heat transfer speed.
Note: The iron / carbon diagram does not take the heat transfer speed into account.
Important metals that have alternative structures depending on the temperature are:
- Ca
- Co
- Hf
- Fe
- Li
- Na
- Ti
- Y
- Zr
Citation
When you need to include a fact or piece of information in an assignment or essay you should also include where and how you found that piece of information (Crystal Systems).
That gives credibility to your paper and it is sometimes required in higher education.
To make your life (and citation) easier just copy and paste the information below into your assignment or essay:
Luz, Gelson. Crystal Systems. Materials Blog. Gelsonluz.com. dd mmmm. yyyy. URL.
Now replace dd, mmmm and yyyy with the day, month, and year you browsed this page. Also replace URL for the actual url of this page. This citation format is based on MLA.













COMMENTS